The Advocate
Informing Marine Engineers about legal matters
Authored by: Darren Williams, Williams & Company
Brought to you by www.dieselduck.net, comments to [email protected]
“Electrolysis” and Impressed Current Corrosion as an insured “Peril of the Sea”
If your vessel has ever been damaged by “electrolysis” and you
have claimed on your hull and machinery insurance, chances are you
have been denied insurance coverage for the damage. Despite the fact
that only some policies of marine insurance specifically exclude
“electrolysis” as a loss insured against, coverage for such damage
is invariably denied because underwriters take the convenient view
that “electrolysis” is a gradual and inevitable process (like wood
rot), and therefore not a sudden and fortuitous event that qualifies
as a “peril of the sea”, that is, a typical insurable loss.
I am currently running two lawsuits against underwriters on this
issue, both with a view to setting precedents whereby underwriters
are bound to accept, as a loss covered by the insurance policy,
damage by some forms of “electrolysis” – these are matters of public
record. In this "Advocate" I will discuss why certain
electrochemical corrosion, particularly “impressed stray current
corrosion”, should (and hopefully will) be a form of damage covered
by typical hull and machinery claims in the near future.
Underwriters should sit down while reading this article.
Coverage for Perils of the Sea under a Hull & Machinery Policy
Hull and machinery policies are “named perils” policies, that is,
the owner (or “assured”) is protected from losses that occur as a
result of perils that are named in the policy. The most common
example of this on the West Coast is the Canadian Hull (Pacific)
Clauses 1991. These insuring clauses, in conjunction with provisions
in the Canadian Marine Insurance Act, set out that losses that occur
as the result of a marine adventure or peril are covered, unless
they are specifically excluded. A “marine peril” is a peril
consequent to marine navigation, and typically refers to damage by
waves, sinking, and so on. Underwriters will often attach to these
general Canadian Hull (Pacific) Clauses other provisions that set
out particular exclusions to coverage, such as “mould”, “vermin” or
“electrolysis” damage. In the two cases referred to above, neither
policy contains a specific exclusion for electrolysis (as discussed
below, even if they did, in my opinion, underwriters may not be able
to deny coverage for some types of electrolysis, such as impressed
current corrosion).
Under the Canadian Hull (Pacific) Clauses, a loss must result from a
“marine peril” or “peril of the sea” to be afforded coverage. A
“peril” has traditionally been construed to only include events that
are “sudden” and “fortuitous” (meaning they are accidental, or not
an inevitable event). To explain by analogy, while you can have life
insurance in the event of a heart attack or stroke (these are sudden
and not inevitable events), you cannot insure against death by old
age generally because this is a gradual and inevitable event.
Those typical exclusions noted above, such as “mold” and “vermin” are generally associated with a failure to maintain the vessel, and not a “sudden” and “fortuitous” loss.
The difficulty with the word “electrolysis” is that it has been
so badly misused over the years, that underwriters simply understand
it to be a gradual and inevitable process, and therefore not a
“peril” that can be insured against (unless of course underwriters
simply use it as an excuse to deny coverage and save a few bucks,
but I can’t imagine that being the case). For example, when one of
my clients received a letter from their insurance broker confirming
the policy would not cover the damage to his boat that occurred over
a period of only a few days and in proximity to a vessel that was
measured to be leaking current, the broker stated:
I refer you to correspondence received form your Insurer dated June
17, 2005. It clearly outlined the reasons for denial. It states “the
surveyor had revealed that the vessel had sustained electrolysis
damage in the form of pin-holing, pitting and patches of surface
erosion over the entire underwater hull”. It referred to insuring
conditions contained in the Canadian Hulls (Pacific ) clauses, 1991
which is a named perils policy. Electrolysis damage over a ‘period
of time’ is not a covered peril as it was not considered ‘sudden and
accidental’.
In my opinion, it is the underwriter’s misconception of
“electrolysis”, and perhaps their motivation to save money by
denying a claim, that results in the erroneous conclusion that the
damage was not sudden and accidental (fortuitous). To this end, a
discussion on this type of corrosion is appropriate.
The Common Misunderstanding of “Electrolysis”
“Electrolysis” is a misused word. In a marine context,
“electrolysis” may most commonly be defined as a chemical change in
a metal that results from the passage of electrons (from one metal
to another) through an electrolyte such as salt water. We most often
see electrochemical corrosion in two forms, galvanic corrosion and
electrolytic corrosion.
Galvanic corrosion will occur when two different metals, each with
their own electrical potential (ability to hold their electrons),
are placed in proximity - the flow of electrons from one metal to
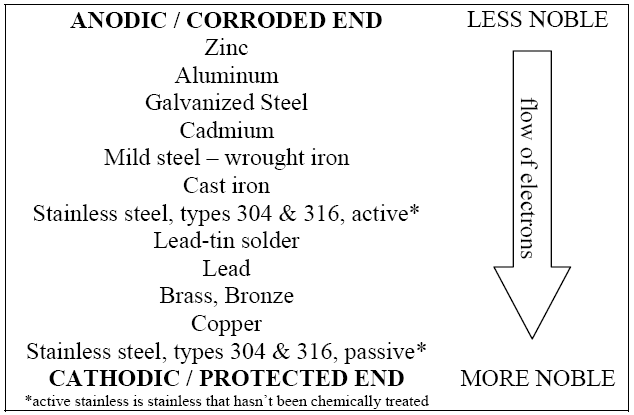
the other results in corrosion to the metal. Figure 1 is a common
table of the galvanic potential of various metals – the further
apart two metals are on the galvanic scale the more vigorously they
will react. Galvanic corrosion is most effective when an
electrolytic solution (such as salt water) is present between the
metals, but can occur when it is dry if the metals are touching.
This type of corrosion tends to be a gradual process, and does not
require an external source of current to occur. It can be found on a
boat when, for example, a steel and a brass pipe fitting, each
having a different potential, are joined (the steel will lose
electrons to the brass, causing the steel to rust).
Electrolytic corrosion, on the other hand, may commonly be
understood to be the loss of metal as electrons flow from one metal
to distant metal through an electrolyte such as salt water. Zincs on
your hull dissolve as electrons pass from the zinc out into the
water and onto other metals. Unlike galvanic corrosion, electrolytic
corrosion can be found even when metals are not tied together or in
close proximity, and will only occur when there is an electrolyte
solution. In normal circumstances, electrolytic corrosion typically
happens relatively slowly – some mariners may replace their zincs
only once every two years or so. Unfortunately, not all
circumstances are “normal”.
Stray Current (Electrolytic) Corrosion - Electrolytic corrosion can
occur much more quickly when there is “stray current” present. Stray
current is current that exists between two substances other than
because of the difference in each metal’s natural potential. Stray
current can occur, for example, when there is a fault in the DC
system of a vessel and the DC current strays, or leaks, through the
vessel and out the hull into the water looking for ground – as it
leaves the hull it takes electrons of metal with it causing the
metal to corrode. Another example is when a vessel, who is plugged
into shore power, has a ground fault in the DC system which allows
DC current to flow into the dock’s ground system – this DC current
will flow back onto each vessel (unless the vessel is protected with
a galvanic isolator or isolation transformer) in the marina that is
plugged into the ground, and out through each of the hulls back to
ground. Again, as this current passes out of the hull it takes
electrons of metal with it, corroding the metal. This corrosion can
be more than zincs can handle. Indeed, corrosion may occur to
portions of the hull even though there is life left in the zincs
because the zincs may be insulated by a thin layer of zinc oxide, or
the zinc has lost a proper connection to the hull.
Impressed Current Corrosion - More severe forms of stray current
corrosions also exist, such as when there is a great deal of stray
current or the voltage associated with the current is high. Because
it is impressed, the current can cause catastrophic damage to the
hull extremely quickly – destroying the hull in a matter of days.
More severe examples of DC current ground faults can cause this. As
well, although AC current is commonly thought not to be able to
cause stray current corrosion, there are some authorities that opine
that metallic oxides can act as diodes that convert the AC current
into DC current at the surface of the metals, and that because the
voltage of AC is much higher than DC, damage caused by AC current
can occur as dramatically and as quickly as that caused by DC
current. There are however conflicting opinion on this point.
In summary, contrary to underwriter’s common position that all
electrochemical corrosion is “electrolysis”, and that electrolysis
is “damage over a ‘period of time” and “not a covered peril as it
was not considered ‘sudden and accidental’”, stray current
corrosion, particularly when associated with impressed current, can
cause sudden and accidental corrosion. Hence, in my opinion, this is
damage that qualifies as a “peril of the sea” and is thus an insured
loss.
In both of the cases referred to above, the vessels were either
found in areas where there was significant stray current recorded in
close proximity to the damage, and the vessel was tied into a common
ground, or there was a significant ground fault in the onboard
system and the vessel had no mechanism to isolate the stray current
from the hull. Both vessels suffered sudden and dramatic damage to
the hull. In both cases, I expect, the underwriter is in for a rude
awakening.